Elucidation of structures and lithium environments for an organo-sulfur cathode†
Abstract
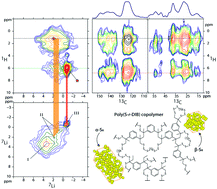
Composite organo-sulfur cathodes provide a unique platform for the realization of lithium–sulfur (Li–S) cells. However, difficulties arise in the interpretation of the function of these electrodes in Li–S cells and the role they play in suppressing the so-called ‘shuttle effect’. This work focuses on monitoring in detail the structural evolution and lithium environments during charge–discharge cycles in a lithium half-cell of an organo-sulfur cathode, which was synthesised by inverse vulcanisation with 1,3-diisopropenylbenzene. For the first-time in organo-sulfur materials, high resolution solid state 7Li–1H and 13C–1H double resonance NMR spectroscopy coupled with X-ray absorption near-edge structure (XANES) and X-ray diffraction (XRD) are used to develop a detailed structural model of the cathode material and its lithium environments as a function of cycle number. This work provides the first experimental evidence via 2D NMR spectroscopy of distinct molecular proximities of the lithium species with respect to the sulfur, the organic skeleton and the electrolyte in the cathode material. This approach enables us to develop unparalleled understanding of the mechanisms of the high charge capacity of 607 mA h g−1, rationalising initial capacity drop and suppression of capacity fade with cycling. These results also show new possibilities on how to better understand electrode function to further increase the lithium capacities of organo-sulfur cathode materials, which can in turn lead to performance-enhanced Li–S cells.


 Please wait while we load your content...
Please wait while we load your content...