An experimental and computational IR and hybrid DFT-D3 study of the conformations of l-lactic and acrylic acid: new insight into the dehydration mechanism of lactic acid to acrylic acid
Abstract
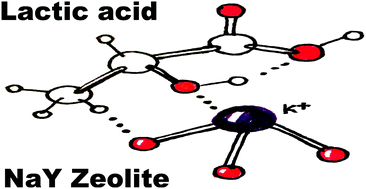
We have studied using hybrid Density Functional Theory (DFT) with an aug-cc-pVTZ basis set and D3 dispersion corrections the intra-molecular hydrogen bond of L-lactic acid and L-lactic-acid analogs with the hydroxyl group on the alpha carbon atom substituted by α-XH (where X = S, Se, Te) as well as the conformations of acrylic acid. The results show that there are three types of intramolecular hydrogen bonds that can form only when α-OH is present, whereas other less electronegative functional groups such as –SH, –SeH and –TeH do not exhibit the formation of an intramolecular H-bond. We show that the intra-molecular H-bond formed between the alpha-OH hydrogen and the COOH carbonyl oxygen would enhance the rate of nucleophilic substitution of alpha-OH at the K+ sites in the previously suggested dehydration mechanism of L-lactic to acrylic acids. We find that a temperature range between 190 and 210 °C would be optimum to maximise the rate of nucleophilic substitution of the alpha-OH group at the potassium sites during the dehydration mechanism of L-lactic acid to acrylic acid. Additionally, our hybrid-DFT simulation of the infrared spectrum of the various conformers shows that the lowest energy conformer can be identified by a single vibrational band at 3734 cm−1 whereas for the other conformers, this vibrational band is split with Δν that ranges between 6 cm−1 and 176 cm−1. We also find that the various conformers of acrylic acid can be identified by a double peak for the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and O–H vibrations, which have Δν′ and Δν′′ values of 24 and 42 cm−1, respectively. This computational study is useful for spectroscopic experimental efforts that try to identify the various conformers of L-lactic acid and acrylic acid and to gain mechanistic insight into the dehydration mechanism over K substituted NaY zeolites.
O and O–H vibrations, which have Δν′ and Δν′′ values of 24 and 42 cm−1, respectively. This computational study is useful for spectroscopic experimental efforts that try to identify the various conformers of L-lactic acid and acrylic acid and to gain mechanistic insight into the dehydration mechanism over K substituted NaY zeolites.


 Please wait while we load your content...
Please wait while we load your content...