Exploration of HIV-1 fusion peptide–antibody VRC34.01 binding reveals fundamental neutralization sites†
Abstract
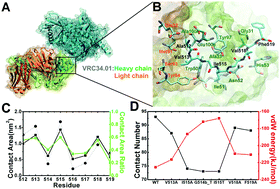
Antibody binding to a vulnerable site of HIV envelope glycoprotein (Env), the eight N-terminal residues of the gp41 fusion peptide, renders robust HIV neutralization. Here, we theoretically investigate HIV-1 fusion peptide binding to the neutralizing antibody N123–VRC34.01. We explore numerous fusion peptide mutations using all-atom molecular dynamics simulation with explicit-solvent models. Simulation results show that the hydrophobic interaction between Ile515 in the HIV-1 fusion peptide and the antibody VRC34.01 Fab plays an important role in antibody binding. Furthermore, we verify by free energy perturbation (FEP) calculations that two point mutations of Ile515Thr or Ile515Ala can dramatically weaken the binding affinity. Our findings provide new insights into fusion peptide–VRC34.01 binding, which can ultimately be utilized to design effective HIV vaccines.


 Please wait while we load your content...
Please wait while we load your content...