Electron transfer and intersystem crossing triggered fluorescence quenching detection of mercury ions†
Abstract
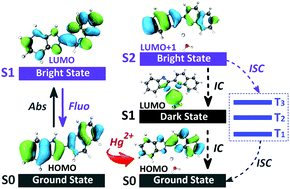
Via a specific fluorescence quenching response, organic compound L serves as an excellent probe for Hg2+ in aqueous solution. The underlying detection mechanism is proposed based on the analyses of orbital interactions between probe L and Hg2+, which is new and intrinsically different from the previously proposed one. By investigating the excitation process and the excited state deactivation process of the organometallic compound formed by L and Hg2+, two non-emissive channels, namely intermolecular electron transfer and intersystem crossing, are observed. The co-effect of the two channels leads to the significant fluorescence quenching of L. Hopefully, the newly proposed mechanism can inspire experimentalists to encapsulate the two channels into one probe to achieve accurate detection of metal ions.


 Please wait while we load your content...
Please wait while we load your content...