Elucidating the mechanism and kinetics of the water-assisted reaction of nitrous acid with hydroxyl radical†
Abstract
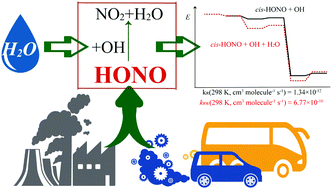
The effect of a single water molecule on the atmospheric reaction between nitrous acid (HONO) and the hydroxyl radical (OH) has been investigated in this work. The CCSD(T)/aug-cc-pVTZ//UωB97X-D/aug-cc-pVTZ level of theory was used to obtain all stationary points on the energy surface and to determine the rate constants. Due to the two HONO configurations (cis- and trans-) existing in the atmosphere, the water-free HONO + OH reaction has two major elementary channels, both based on the HONO hydrogen abstraction by the hydroxyl radical. The products, NO2 + H2O, are formed with substantial energy gain, but separated by relatively low energy barriers. In the presence of water, the reaction becomes more complex, proceeding through twelve different channels, each starting with the formation of a binary complex between water and one reactant followed by its interaction with the third species. The products are similar to those of the water-free reaction. At 298 K, the rate constants of water-free cis-HONO + OH and trans-HONO + OH reactions are 1.34 × 10−12 and 1.00 × 10−15 cm3 molecule−1 s−1, respectively. The calculated rate constants for H2O-complexed HONO or OH increase by one to two orders of magnitude, but weighted by their relative abundances, the H2O-complexed fractions of the reactants in the atmosphere are so small that the effect of H2O on the overall reaction rate is minor.


 Please wait while we load your content...
Please wait while we load your content...