Electronic and magnetic properties of phosphorene tuned by Cl and metallic atom co-doping
Abstract
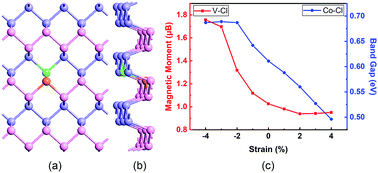
Using first-principles calculations based on density functional theory, we studied the electronic and magnetic properties of phosphorene co-doped with Cl and a metal atom (including Sc, Ti, V, Cr, Mn, Fe, Co, and Ni). It is found that Cl atom doping makes it much easier for metallic atoms to dope into phosphorene. Phosphorene co-doped with Cl and V, Cr, Mn, or Fe is magnetic, which is determined by the number of valence electrons. Taking V–Cl and Co–Cl co-doped phosphorene as an example, analyses are carried out on the reasonable selection of the doping sites, which distinctly affect the stability, band gap and magnetic moment. The stability is closely relevant to the electronegativity of impurity atoms. With the biaxial strain ranging from −4% to 4%, the magnetic moment of V–Cl co-doped phosphorene and the band gap of Co–Cl co-doped phosphorene are greatly tunable between 1.757–0.951 μB and 0.687–0.496 eV, which come from the electron transfer from V to the surrounding P atoms and the weakened bond between Co and Cl, respectively. These investigations provide a reference for regulating the electronic structure and magnetic properties of diluted magnetic semiconductors and promote the applications of phosphorene in spintronics and nanodevices.


 Please wait while we load your content...
Please wait while we load your content...