It's not just the defects – a curved crystal study of H2O desorption from Ag
Abstract
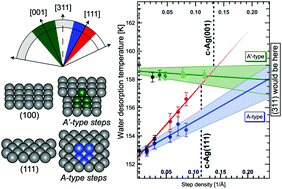
We investigate water desorption from hydrophobic surfaces using two curved Ag single crystals centered at (111) and (001) apices. On these types of crystals the step density gradually increases along the curvature, allowing us to probe large ranges of surface structures in between the (001), (111) and (110) planes. Subtle differences in desorption of submonolayer water coverages point toward structure dependencies in water cluster nucleation. The B-type step on hydrophobic Ag binds water structures more strongly than adjacent (111) planes, leading to preferred desorption from steps. This driving force is smaller for A-type steps on (111) terraces. The A′-type step flanked by (001) terraces shows no indication of preferred desorption from steps. Extrapolation to the (311) surface, not contained within either curved surface, demonstrates that both A- and A′-type steps can be regarded chemically identical for water desorption. The different trends in desorption temperature on the two crystals can thus be attributed to stronger water adsorption at (001) planes than at (111) planes and identical to adsorption at the step. These results show that our approach to studying the structure dependence of water desorption is sensitive to variations in desorption energy smaller than ‘chemical accuracy’, i.e. 1 kcal mol−1.
- This article is part of the themed collections: PCCP Editor’s Choice, 2020 and 2019 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...