Experimental and computational approaches to rationalise multicomponent supramolecular assemblies: dapsone monosolvates†
Abstract
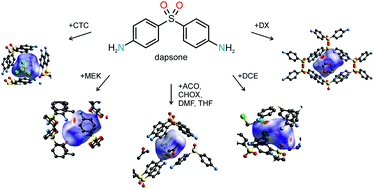
The monosolvate crystal energy landscapes of dapsone (DDS) including the solvents carbon tetrachloride, acetone, cyclohexanone, dimethyl formamide, tetrahydrofuran, methyl ethyl ketone, 1,2-dichloroethane, 1,4-dioxane, dichloromethane and chloroform were established using experimental and computational approaches. To rationalise and understand solvate formation, solvate stability and desolvation reactions a careful control of the experimental crystallisation and storage conditions, a range of thermoanalytical methods and crystal structure prediction were required. Six of the eight DDS monosolvates are reported and characterised for the first time. Structural similarity and diversity of the at ambient conditions unstable monosolvates were apparent from the computed crystal energy landscapes, which had the experimental packings as lowest energy structures. The computed structures were used as input for Rietveld refinements and isostructurality of four of the monosolvates was confirmed. Packing comparisons of the solvate structures and molecular properties of the solvent molecules indicated that both size/shape of the solvent molecule and the possible DDS⋯solvent interactions are the important factors for DDS solvate formation. Through the combination of experiment and theory solvate stability and structural features have been rationalised.


 Please wait while we load your content...
Please wait while we load your content...