Electronic structure modifications induced by increased molecular complexity: from triphenylamine to m-MTDATA
Abstract
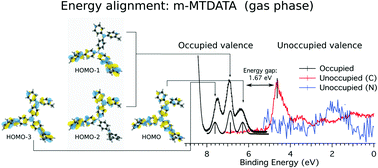
The starburst π-conjugated molecule 4,4′,4′′-tris(N-3-methylphenyl-N-phenyl-amino)triphenylamine (C57H48N4, m-MTDATA), based on triphenylamine (TPA) building blocks, is widely used in optoelectronic devices due to its good electron-donor characteristics. The electronic structure of m-MTDATA was investigated for the first time in the gas phase by means of PhotoElectron Spectroscopy (PES) and Near Edge X-ray Absorption Fine Structure (NEXAFS) spectroscopy. The combination of Density Functional Theory (DFT) calculations with the experimental spectra provides a comprehensive description of the molecular electronic structure. Moreover, by comparing the results with previous TPA measurements, we could shed light on how the electronic structure evolves when the molecular size is increased. We found that the C 1s photoelectron spectra of m-MTDATA and TPA are similar, due to the balance of the counter-acting effects of the electronegativity of the N atoms and the delocalization of the amine lone-pair electrons. In contrast, the increased number of N atoms (i.e. N lone pairs) in m-MTDATA determines a three-peak feature in the outermost valence binding energy region with strong contributions by the N 2pz orbitals. We also obtained a decrease of the HOMO–LUMO gap for m-MTDATA, which points to improved electron donating properties of m-MTDATA with respect to TPA.


 Please wait while we load your content...
Please wait while we load your content...