Tunable pH-dependent oxygen evolution activity of strontium cobaltite thin films for electrochemical water splitting†
Abstract
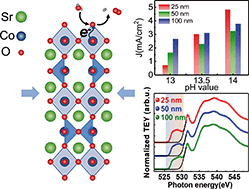
Understanding the oxygen evolution reaction (OER) dependence on the reaction environment pH is important to find alternative strategies to define an optimal pH value for high electrocatalytic activity. SrCoO2.5 films with the brownmillerite phase are investigated in this study for their strain effects on the OER activity, with particular regard to the pH dependence. Pulsed laser deposited films with different thicknesses and, thus, strain conditions, are characterized in terms of long range and near-order structural properties and electrochemical OER activity. By comparison, more strained thinner films have smaller OER current at lower pH conditions, but higher sensitivity to the environment pH. Spectroscopic measurements allow us to correlate such behaviors to the Co 3d–O 2p hybridization effects of the CoO6 octahedral sites, which lead to a variation of the 3d level electronic occupation. At the same time, density functional theory calculations show that the oxygen vacancy channels of the CoO4 tetrahedral sites are stable with respect to the strain effects. These results provide new perspectives to manipulate the pH dependent OER activity through the strain effects, useful for designing water splitting-based devices with optimized performances.


 Please wait while we load your content...
Please wait while we load your content...