The catalytic mechanism of S-acyltransferases: acylation is triggered on by a loose transition state and deacylation is turned off by a tight transition state†
Abstract
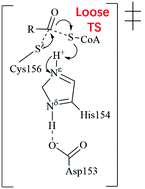
Dynamic protein S-palmitoylation catalyzed by S-acyltransferases is one of the fundamental post-translational modifications involved not only in a wide range of vital cellular processes but also in a series of human health and diseases-related issues (such as brain development and behavior, immune response regulation, tumor suppressor, and cancer). Interestingly, human S-acyltransferase has been recognized as a promising drug-target for cancer treatment. Despite the prominent importance, the fundamental catalytic mechanism of S-acyltransferases remains elusive. In this study, we performed extensive simulations and calculations to describe the fundamental catalytic mechanism of autoacylation catalyzed by human S-acyltransferase, revealing a single-step reaction pathway characterized by a loose transition state. The deacylation process with a tight transition state is avoided due to the substantially high free energy barrier. This specific catalytic mechanism is necessary to protect palmitoylated-enzymes from unwanted hydrolysis by water molecules since the accumulated negative charge of the tight transition state cannot be effectively stabilized by the weak hydrogen bond of the oxyanion hole. The activation free energy barrier of the autoacylation catalyzed by human S-acyltransferase was predicted to be 17.8 kcal mol−1, which is in good agreement with the experimentally derived activation free energy barrier (18.5 kcal mol−1) based on the conventional transition state theory, suggesting the validity of the computational results. The mechanistic insights (e.g., detailed catalytic mechanism and the nature of the transition state) are expected to do good not only to rational drug discovery (e.g., design of transition state analogues) toward cancer treatment and the design of engineered S-acyltransferases with desired acyl-CoA specificity but also to future studies on the catalytic mechanisms of other S-acyltransferases.
- This article is part of the themed collection: 2019 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...