Electron spectroscopy of ionic liquids: experimental identification of atomic orbital contributions to valence electronic structure†
Abstract
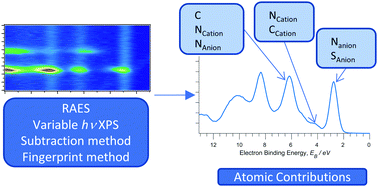
The atomic contributions to valence electronic structure for 37 ionic liquids (ILs) are identified using a combination of variable photon energy XPS, resonant Auger electron spectroscopy (RAES) and a subtraction method. The ILs studied include a diverse range of cationic and anionic structural moieties. We introduce a new parameter for ILs, the energy difference between the energies of the cationic and anionic highest occupied fragment orbitals (HOFOs), which we use to identify the highest occupied molecular orbital (HOMO). The anion gave rise to the HOMO for 25 of the 37 ILs studied here. For 10 of the ILs, the energies of the cationic and anionic HOFOs were the same (within experimental error); therefore, it could not be determined whether the HOMO was from the cation or the anion. For two of the ILs, the HOMO was from the cation and not from the anion; consequently it is energetically more favourable to remove an electron from the cation than the anion for these two ILs. In addition, we used a combination of area normalisation and subtraction of XP spectra to produce what are effectively XP spectra for individual ions; this was achieved for 10 cations and 14 anions.
- This article is part of the themed collection: 2019 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...