Resonant photoionization of O2 up to the fourth ionization threshold†
Abstract
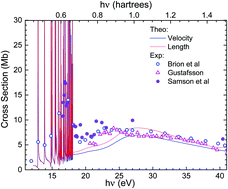
We present a detailed theoretical study of valence-shell photoionization of the oxygen molecule by using the recently proposed XCHEM method. This method makes use of a hybrid Gaussian and B-spline basis in the framework of a close-coupling approach to describe electron correlation in the molecular electronic continuum at a level comparable to that provided by multi-reference configuration interaction methods in bound state calculations. The computed total and partial photoionization cross sections are presented and discussed, with emphasis on the series of autoionizing resonances that appear between the first and the fourth ionization thresholds, i.e., photon energies between 12 and 18 eV. More than fifty autoionizing states are identified, including series not previously reported in the literature, and their energy positions and widths are provided. The present results illustrate the potential of the XCHEM approach to accurately describe molecular autoionization, which is mostly due to electron correlation. This is relevant in view of current experimental efforts aimed at providing real-time (attosecond) imaging of autoionization dynamics in molecules.
- This article is part of the themed collection: 2019 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...