Fluorinated alkyl-phosphate-based electrolytes with controlled lithium-ion coordination structure†
Abstract
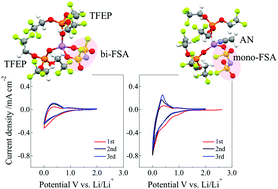
Herein, we propose Li-ion solvation-controlled electrolytes based on non-flammable organic solvent TFEP and an LiFSA salt [TFEP: tris(2,2,2-trifluoroethyl)phosphate, LiFSA: lithium bis(fluorosulfonyl)amide] to allow Li-ion insertion into a graphite electrode for Li-ion batteries. Comprehensive structural study based on (1) infrared (IR)/Raman spectroscopy, (2) high-energy X-ray total scattering (HEXTS), and (3) molecular dynamics (MD) simulation revealed the solvation (or coordination) structures of Li ions in TFEP-based electrolytes at the molecular level. In binary LiFSA/TFEP with a Li salt concentration (cLi) < 1.0 mol dm−3, Li ions are coordinated with both TFEP and FSA components; in detail, two TFEP molecules coordinate in an O-donating monodentate manner and one FSA in an O-donating bidentate manner to form [Li(TFEP)2(bi-FSA)] as the major species. We demonstrated that adding acetonitrile (AN) to the LiFSA/TFEP electrolytes caused structural changes in the Li-ion complexes. The bi-FSA bound to the Li ion changed its coordination mode to mono-FSA, which was induced by solvating AN molecules to Li ions. The redox reaction corresponding to insertion/deinsertion of Li ions into/from the graphite electrode successfully occurred in 1.0 mol dm−3 LiFSA/TFEP with an AN electrolyte system, while there was no or reduced Li-ion insertion in the electrolyte without AN. We discussed the relationship between the structure and electrode reaction of the Li-ion complexes based on the FSA-coordination characteristics; i.e., in LiFSA/TFEP with the AN system, the mono-FSA bound to the Li ion is easier to decoordinate due to weaker Li+⋯mono-FSA− interactions rather than the Li+⋯bi-FSA− interactions, which mainly contribute to charge-transfer at the electrode/electrolyte interface to allow Li-ion insertion/deinsertion in the graphite anode.


 Please wait while we load your content...
Please wait while we load your content...