Elusive hypervalent phosphorus⋯π interactions: evidence for paradigm transformation from hydrogen to phosphorus bonding at low temperatures†
Abstract
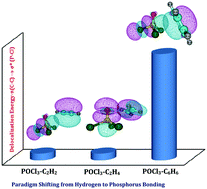
The π electron systems are the conventional electron donors to the hydrogen acceptors in hydrogen bonding. Apart from the hydrogen atom, halogens, chalcogens, pnicogens and triel/tetrel atoms can also be envisaged as electron acceptors involving π clouds. Markedly, in pnicogen⋯π interactions, the bonding of the hypervalent (predominantly pentavalent) state of the phosphorus atom with π electron donors is elusive and can be thought of as an intuitive extension to trivalent phosphorus⋯π interactions. In this work, on the one hand, POCl3 was taken as a prototypical molecule to explore these pentavalent phosphorus interactions and on the other hand, acetylene (C2H2), ethylene (C2H4) and benzene (C6H6), in which phosphorus⋯π bonding can be expected to compete with hydrogen and halogen bonding interactions, were taken as π electron donors. All three POCl3–C2H2, POCl3–C2H4 and POCl3–C6H6 heterodimers were experimentally generated at low temperatures in Ar and N2 matrices and were characterized by both infrared spectroscopy and state-of-the-art quantum chemical computations. Though hydrogen bonding dominates in POCl3–C2H2 and POCl3–C2H4 heterodimers, phosphorus bonding plays a definite and non-trivial role in their overall stabilization. An interesting paradigm transformation was noticed in the POCl3–C6H6 system, where pentavalent phosphorus⋯π bonding was observed to completely influence the hydrogen bonding interaction. To further shed light on these P⋯π systems, the interaction characteristics were analyzed with the help of electrostatic potential mapping, natural bond orbital and energy decomposition analyses.
- This article is part of the themed collection: 1st International Conference on Noncovalent Interactions


 Please wait while we load your content...
Please wait while we load your content...