Effects and distribution of Zr introduced in Ni-based cathode material for Li-ion batteries†
Abstract
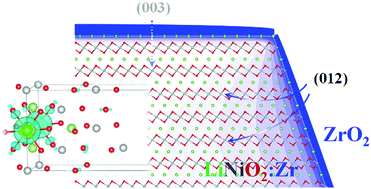
The spatial and site distribution of Zr introduced to the LiNiO2 (LNO) host material and the related mechanism of performance improvement as a Li-ion battery electrode are theoretically investigated. It is found that the equilibrium doping limit of Zr is higher near the (012) surface than that near (003) among the main surface planes of LNO, and that the diffusion energy barrier in the [012] direction is lower than that in [003]. The thermodynamic and kinetic aspects indicate the main Zr diffusion pathway into crystalline LNO via the (012) surface in the perpendicular direction and its crystallographic equivalents. Regarding the site-distribution in the bulk crystal, Zr preferentially substitutes Ni, but becomes located at Li sites as the composition of the LNO host becomes Li-deficient. It is observed that Zr doping suppresses O release from the LNO surface, thus suppressing side reactions at the cathode–electrolyte interface. This is attributed to the mechanism of increased covalent and electrostatic interactions with the O ions by the introduction of Zr. The pillar effect of Zr is achievable with a suitable Li-deficient composition during heating, which induces Zr localization at Li sites. The distribution of Zr is found to be determined by the physical and chemical factors of the doping site size and the chemical bonding characteristics of Zr with neighboring O ions.


 Please wait while we load your content...
Please wait while we load your content...