Vibrational spectroscopy of the hexahydrated sulfate dianion revisited: role of isomers and anharmonicities†
Abstract
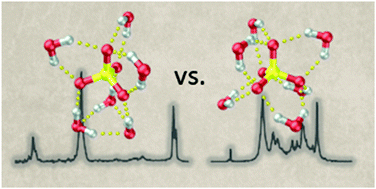
We report on the gas phase vibrational spectroscopy of the hexahydrated sulfate dianion, SO42−(H2O)6, and its fully deuterated isotopologue, SO42−(D2O)6, using infrared photodissociation (IRPD) spectroscopy of the D2-tagged dianions in combination with density-functional-theory calculations on minimum-energy structures as well as finite temperature ab initio molecular dynamics (AIMD) simulations. The IRPD spectra were recorded at an ion trap temperature of 12 K and in the spectral range from 650 to 3800 cm−1, covering the intramolecular modes of the solvent (OH/OD stretches and H2O/D2O bends) at higher energies, those of the solute (sulfate stretches) at intermediate energies and the intermolecular solute librational modes at the lowest energies. Isomer-specific double resonance in combination with messenger-tag dependent IRPD spectra show that only a single isomer is contributing significantly and that this isomer is not the highly symmetric Td but rather the lower symmetry C3 isomer. Temperature-dependent IR multiple photon dissociation spectra of bare SO42−(H2O)6 suggest that the C3 isomer remains the most stable one up to 200 K. The AIMD simulations reveal that the IRPD spectra can only be fully understood when anharmonic effects as well as entropy-driven hydrogen bond network fluctuations are considered.
- This article is part of the themed collection: 2019 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...