Structural and functional role of anions in electrochemical water oxidation probed by arsenate incorporation into cobalt-oxide materials†
Abstract
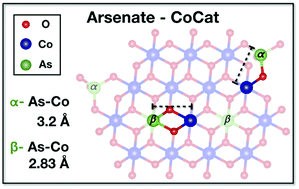
Direct (photo)electrochemical production of non-fossil fuels from water and CO2 requires water-oxidation catalysis at near-neutral pH in the presence of appropriate anions that serve as proton acceptors. We investigate the largely enigmatic structural role of anions in water oxidation for the prominent cobalt-phosphate catalyst (CoCat), an amorphous and hydrated oxide material. Co3([(P/As)O]4)2·8H2O served, in conjunction with phosphate–arsenate exchange, as a synthetic model system. Its structural transformation was induced by prolonged operation at catalytic potentials and probed by X-ray absorption spectroscopy not only at the metal (Co), but for the first time also at the anion (As) K-edge. For initially isostructural microcrystals, anion exchange determined the amorphization process and final structure. Comparison to amorphous electrodeposited Co oxide revealed that in CoCat, the arsenate binds not only at oxide-layer edges, but also arsenic substitutes cobalt positions within the layered-oxide structure in an unusual AsO6 coordination. Our results show that in water oxidation catalysis at near-neutral pH, anion type and exchange dynamics correlate with the catalyst structure and redox properties.


 Please wait while we load your content...
Please wait while we load your content...