Dynamical fluxionality, multiplicity of structural forms, and electronic properties of the B3Si11 cluster: anion photoelectron spectroscopy and theoretical calculations†
Abstract
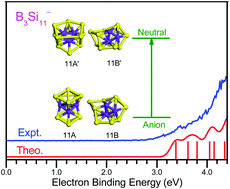
The geometrical structures and electronic properties of anionic, neutral, and cationic B3Si11 clusters were investigated by performing ab initio calculations combined with size-selected anion photoelectron spectroscopy. The experimental photoelectron spectrum of the B3Si11− anion is reasonably reproduced by theoretical simulations of two competing isomers. The global minimum of the B3Si11− anion is formed by the fusion of a B3Si7 bicapped tetragonal antiprism to a B3Si4 pentagonal bipyramid by sharing a B3 triangle, while that of neutral B3Si11 has a B3-endohedral sandwich structure composed of a Si5 five-membered ring and a Si6 six-membered ring, and that of the B3Si11+ cation adopts a Si11 tricapped tetragonal antiprism with three face-capping B atoms. It is interesting that a Si5 five-membered ring and a Si6 six-membered ring are stabilized by three B atoms in B3Si11. The three B atoms tend to bond with each other to form a B3 triangle with stronger B–B bonds than B–Si bonds. Moreover, neutral B3Si11 exhibits σ + π double delocalized bonding patterns. Anionic, neutral, and cationic B3Si11 clusters have multiplicity of structural forms and their low-lying isomers show dynamical fluxionality. The bond lengths, bond orders, MO, constant electronic charge density surfaces, and PDOS analyses showed that the three B atoms in B3Si11 have strong bonding interactions.


 Please wait while we load your content...
Please wait while we load your content...