A novel folding pathway of the villin headpiece subdomain HP35†
Abstract
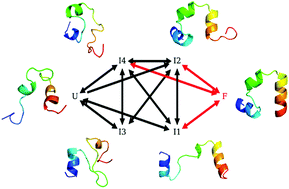
The villin headpiece subdomain (HP35) is a fast-folding protein with 35 residues and its folding pathways have been extensively studied experimentally and theoretically but remain controversial. While experiments showed that HP35 might have multiple folding pathways, most theoretical studies only found one major pathway, although a few theoretical studies revealed two. Here we report our results of molecular dynamics simulations of HP35 folding by using the newest AMBER ff14SB force field and show that HP35 has a novel folding pathway in addition to the two pathways shown previously. We also study the mechanism of determining the folding pathways and found that the dynamics of Helix2 may play a special role in the folding of HP35. Our results may be helpful to understand the folding mechanism of HP35 further.


 Please wait while we load your content...
Please wait while we load your content...