The folding equilibria of enterobactin enantiomers and their interaction with actinides†
Abstract
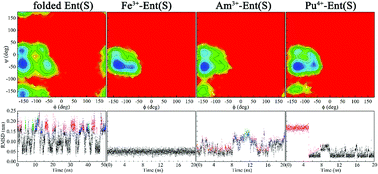
Enterobactin (Ent) is a typical siderophore with strong iron affinity. Its dynamics in its intact form and holo state remains to be studied to understand its role in the in vivo behavior of metal ions and to facilitate its potential application in drug design and environmental remediation. Here, we report molecular dynamics simulations of both Ent enantiomers and their complexes with key actinides (Am3+, Cm3+, Th4+, U4+, Np4+ and Pu4+) to study the folding equilibria of Ent enantiomers and their binding affinity with actinides. For comparison, the ferric cation was also considered. In their neutral state, both enantiomers may exist in their folded and extended states in the aqueous phase with the former more stable owing to the favorable cation–π, π–π, and H-bond interactions. A helicity preference was observed in the folded states of Ent enantiomers, which was solidified when binding with Fe3+ while disrupted when binding with actinides. Upon binding with metal ions, the dynamics of Ent enantiomers exhibited dependence on the metal ions, and appeared to be more flexible in An3+/4+–Ent complexes than in Fe3+–Ent complexes. The conformational analysis and the energy decomposition of M3+/4+–Ent complexes indicated that their distinct conformational variations and dynamic fluxionality are enthalpy driven behaviors and dependent on the nature of the loaded metal ions. The Fe3+–Ent complexes had a more compact conformation, while the relatively loosely bound An3+/4+–Ent complexes allowed solvent water molecules to access the first coordination shell of An3+/4+ and weaken the interaction between An3+/4+ and Ent. This work is expected to enrich our knowledge of the folding equilibria of Ent enantiomers and their An3+/4+–Ent complexes, and contribute to communities that concern the in vivo and in vitro behaviors of Ent enantiomers and actinides.
- This article is part of the themed collection: 2019 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...