Insight into conformationally-dependent binding of 1-n-alkyl-3-methylimidazolium cations to porphyrin molecules using quantum mechanical calculations†
Abstract
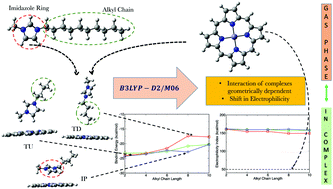
The first step in the biodegradation of imidazolium-based ionic liquids involves the insertion of the –OH group into the alkyl side chain, and it is believed to be triggered by cytochrome P450. However, at present, there is a lack of fundamental understanding of why the hydroxylation process is observed only for longer alkyl chain analogues. As the initial step of the hydroxylation reaction involves the ionic liquid binding to Fe-porphyrin (FeP) – the catalytic center of cytochrome P450, the orientation of ionic liquids presented to FeP is expected to play a crucial role in eventual hydroxylation of the alkyl side chain. In order to elucidate the chain-length dependent binding preferences exhibited by the homologous series of 1-n-alkyl-3-methylimidazolium (n = 2, 4, 6, 8, and 10) [Cnmim]+ cations, a quantum mechanical treatment of the cations in the presence of free base porphyrin (FBP) and FeP is carried out at the B3LYP-D2 and M06 levels. The binding energy of different complexes with FBP and FeP is investigated by considering three vastly different starting relative orientations of the cations with respect to FBP and FeP: tail down, tail up, and interplanar. Our calculations of binding energies reveal that the cation orientations initiated from the tail down conformations (alkyl chain facing the porphyrin molecules) are progressively destabilized as the alkyl chain length increases. The decomposition of the binding energies into various energetic contributions shows that the interaction energy between the cations and porphyrin molecules varies with the cation geometries presented to porphyrin molecules and is the primary determinant of the magnitude of the binding energies. We further demonstrate that the propensity of the cation–FeP complexes to acquire an electron, the next step in the hydroxylation reaction cycle upon substrate binding, is favored independent of the cations and conformations, suggesting that this step is not the reason for the low biodegradability of short alkyl chain bearing cations. Furthermore, the weaker binding of the ionic liquid to FeP is anticipated to facilitate dioxygen binding to FeP, the step following the electron transfer reaction. Overall, the results of the present calculations indicate that the destabilization of the tail down conformations relative to the other two conformations correlates with the experimental results of the chain length-dependent biodegradation of imidazolium-based ionic liquids.


 Please wait while we load your content...
Please wait while we load your content...