Structural analysis of the initial lithiation of NiO thin film electrodes†
Abstract
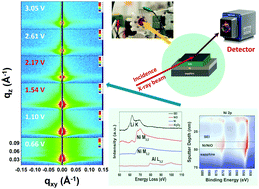
Observations of the initial lithiation of NiO electrodes demonstrate how to seed conversion reactions using interfaces in a thin film Ni/NiO bilayer architecture. Operando X-ray reflectivity (XRR) reveals that structural changes in a NiO film begin at potentials near the theoretical reduction potential (1.8–2.0 V) with detectable lithiation of both the buried Ni/NiO interface and the outer NiO surface that occur prior to the reaction of the NiO film. This initial conversion reaction is most pronounced in ultrathin NiO films (∼20 Å) with only small changes to the NiO film surface for thicker films (∼67 Å). The limited reactivity of thicker NiO films probed using operando grazing incidence small-angle X-ray scattering (GISAXS) shows the growth of nanoparticles at the electrode/electrolyte interface during initial lithium ion insertion, with a 16–20 Å average radius. Ex situ X-ray photoelectron spectroscopy (XPS), time-of-flight secondary ion mass spectrometry (ToF-SIMS), and scanning transmission electron microscopy/electron energy loss spectroscopy (STEM/EELS) confirm our conclusions about the morphological changes accompanying initial stage of lithiation in these conversion reaction electrodes. The present study reveals the interconnected challenges of solid–solid transitions, overpotentials, interfacial nucleation and kinetics, and transition metal dissolution in conversion-type electrodes that are critical for their use as electrodes in lithium-ion batteries.


 Please wait while we load your content...
Please wait while we load your content...