Palladium-catalysed alkyne alkoxycarbonylation with P,N-chelating ligands revisited: a density functional theory study†‡
Abstract
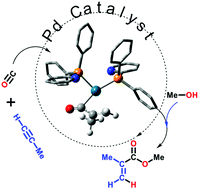
A revised in situ base mechanism of alkyne alkoxycarbonylation via a Pd catalyst with hemilabile P,N-ligands (PyPPh2, Py = 2-pyridyl) has been fully characterised at the B3PW91-D3/PCM level of density functional theory. Key intermediates on this route are acryloyl and η3-propen-1-oyl complexes that readily undergo methanolysis. With two hemilabile P,N-ligands and one or both of them protonated, the overall computed barrier is 16.8 kcal mol−1. This new mechanism is consistent with all of the experimental data relating to substituent effects on relative reaction rates and branched/linear selectivities, including new results on the methoxycarbonylation of phenylacetylene using (4-Me2N-Py)PPh2 and (6-Cl-Py)PPh2 ligands. This ligand is found to decrease catalytic activity over PyPPh2, thus invalidating a formerly characterised in situ base mechanism.


 Please wait while we load your content...
Please wait while we load your content...