Response of microbial membranes to butanol: interdigitation vs. disorder†
Abstract
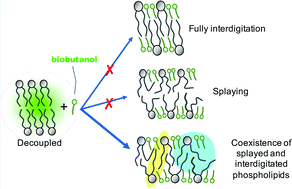
Biobutanol production by fermentation is potentially a sustainable alternative to butanol production from fossil fuels. However, the toxicity of butanol to fermentative bacteria, resulting largely from cell membrane fluidization, limits production titers and is a major factor limiting the uptake of the technology. Here, studies were undertaken, in vitro and in silico, on the butanol effects on a representative bacterial (i.e. Escherichia coli) inner cell membrane. A critical butanol : lipid ratio for stability of 2 : 1 was observed, computationally, consistent with complete interdigitation. However, at this ratio the bilayer was ∼20% thicker than for full interdigitation. Furthermore, butanol intercalation induced acyl chain bending and increased disorder, measured as a 27% lateral diffusivity increase experimentally in a supported lipid bilayer. There was also a monophasic Tm reduction in butanol-treated large unilamellar vesicles. Both behaviours are inconsistent with an interdigitated gel. Butanol thus causes only partial interdigitation at physiological temperatures, due to butanol accumulating at the phospholipid headgroups. Acyl tail disordering (i.e. splaying and bending) fills the subsequent voids. Finally, butanol short-circuits the bilayer and creates a coupled system where interdigitated and splayed phospholipids coexist. These findings will inform the design of strategies targeting bilayer stability for increasing biobutanol production titers.


 Please wait while we load your content...
Please wait while we load your content...