Two solvent-induced variable host–guest two-dimensional binary frameworks mediated by hydrogen bonding†
Abstract
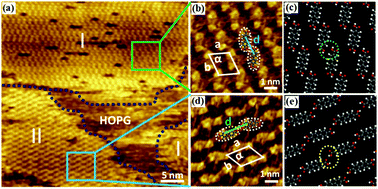
Two-dimensional binary hydrogen-bonded organic frameworks constructed from 1,3,5-benzenetricarboxylic acid (TMA) and 4,4′-biphenyldicarboxylic acid (BDA) on highly oriented pyrolytic graphite (HOPG) were investigated by scanning tunneling microscopy (STM) in heptanoic acid and octanoic acid solvents. High-resolution STM observations demonstrated that various assemblies of hydrogen-bonded networks are strongly dependent on the nature of the solvent. Well-ordered porous rectangular flowerlike networks were revealed at the heptanoic acid/HOPG interface, whereas two different coexisting densely packed guest–host BDA/TMA structures were observed at the octanoic acid/HOPG interface. It is suggested that the stabilization of the binary networks is possibly associated with the solvent chain length, and longer-chain solvents favored the formation of porous polymorphic networks.


 Please wait while we load your content...
Please wait while we load your content...