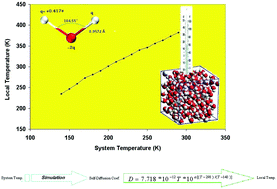
Local temperature versus system temperature in a simulation experiment containing water molecules
Abstract
Water models are commonly used in simulation experiments containing water molecules. However, the geography, electrical properties, and non-bonded interaction parameters provided by these models do not completely fit the experimental data of water molecules. Therefore, these models cannot reproduce the real physical properties of water. We argue that during the simulation experiment, the faults in a water model can cause the water molecules to experience a local temperature that is different from the system temperature. Hence, the calculated physical properties should be related to the local temperature rather than to the system temperature. We have derived a new relation between the temperature and the self-diffusion coefficient of water. We proposed the use of the self-diffusion coefficient of water calculated from the trajectory for obtaining the local temperature in a simulation experiment. This approach was applied to a TIP3P water model. We carried out a series of molecular dynamics simulations of water molecules by this model and plotted the calculated density versus the calculated local temperature. This plot shows a maximum for water density. We also performed another series of MD simulation experiments and plotted the calculated surface tension of water obtained from the literature versus the calculated local temperature. The obtained plot was very close to the experimental plot and predicted a value of 652 K for the critical temperature of water.


 Please wait while we load your content...
Please wait while we load your content...