Vibronic interaction in CO3− photo-detachment: Jahn–Teller effects beyond structural distortion and general formalisms for vibronic Hamiltonians in trigonal symmetries†
Abstract
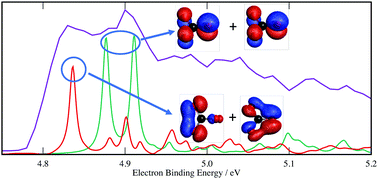
Recently, the negative ion photoelectron spectrum of CO3− was reported and the second lowest energy band is assigned to the close-lying 3E′′ and 3E′ states that undergo Jahn–Teller distortions (Chem. Sci., 2016, 7, 1142). This assignment is based on the Born–Oppenheimer approximation and the assumption of a static Jahn–Teller effect that distorts the CO3 structure from D3h to C2v symmetry. In this work, we employ a 4 states 6 modes vibronic coupling model to investigate the triplet band and uncover the dynamic and non-adiabatic nature of the Jahn–Teller and pseudo-Jahn–Teller interactions in the triplet states. The apparent four peaks progression in the band is studied in depth, and is found to consist of more than four transitions. By comparing the simulated spectra using the full model and the reduced-dimension 2 states 2 modes models, we characterize those transitions. The origin of the complexities of the spectrum is traced to the C–O nonbonding character of the orbitals that lose electron in the photo-detachment process. Methodology-wise, we derive and present the formalisms for arbitrary order expansions of all bimodal trigonal Jahn–Teller and pseudo-Jahn–Teller Hamiltonians in vibrational coordinates.
- This article is part of the themed collection: 2019 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...
