Formation of Criegee intermediates and peroxy acids: a computational study of gas-phase 1,3-cycloaddition of ozone with catechol†
Abstract
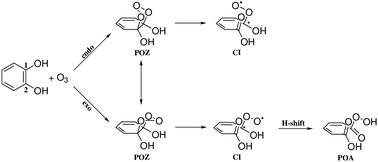
A detailed theoretical investigation of gas-phase 1,3-cycloaddition of ozone with catechol is presented to explore the discrepancies in previous theoretical and experimental rate constants. DFT based PBE, TPSS, B3LYP, B3PW91, M06-2X, wB97XD, MN15 and high-level CCSD(T) methods are used for the calculation. Canonical transition state theory has been used to calculate the rate coefficients of individual steps. The calculated rate coefficients are compared with the experimental and previously calculated rate constant. The possible pathways for primary ozonide (POZ) formation and subsequent reactions to yield the Criegee Intermediates (CI) and peroxy acids (POA) are investigated. The endo-POZ may undergo conversion to exo-POZ or form the Creigee Intermediates. This work shows a novel pathway by which the exo-POZ can form more stable and chemically different species, peroxy acids, by abstracting an H atom from the OH group.


 Please wait while we load your content...
Please wait while we load your content...