Kinetics and equilibrium constants of oligonucleotides at low concentrations. Hybridization and melting study†
Abstract
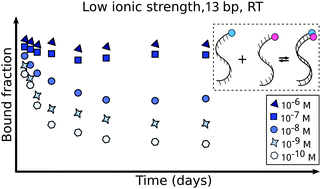
Although DNA hybridization/melting is one of the most important biochemical reactions, the non-trivial kinetics of the process is not yet fully understood. In this work, we use Förster resonance energy transfer (FRET) to investigate the influence of temperature, ionic strength, and oligonucleotide length on the kinetic and equilibrium constants of DNA oligonucleotide binding and dissociation. We show that at low reagent concentrations and ionic strength, the time needed to establish equilibrium between single and double strand forms may be of the order of days, even for simple oligonucleotides of a length of 20 base pairs or less. We also identify and discuss the possible artifacts related to fluorescence-based experiments conducted in extremely dilute solutions. The results should prove useful for the judicious design of technologies based on DNA-matching, including sensors, DNA multiplication, sequencing, and gene manipulation.


 Please wait while we load your content...
Please wait while we load your content...