Understanding the interdependence of operating parameters in microbial electrosynthesis: a numerical investigation†
Abstract
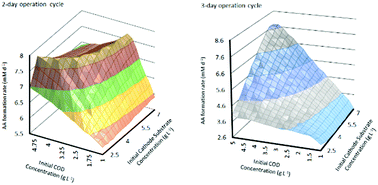
This study describes and evaluates a dynamic computational model for a two chamber microbial electrosynthesis (MES) system. The analysis is based on redox mediators and a two population model, describing bioelectrochemical kinetics at both anode and cathode. Mass transfer rates of the substrate and bacteria in the two chambers are combined with the kinetics and Ohm's law to derive an expression for the cell current density. The effect of operational parameters such as initial substrate concentration at the anode and cathode and the operation cycle time on MES performance is evaluated in terms of product formation rate, substrate consumption and coulombic efficiency (CE). For a fixed operation cycle time of 3 or 4 days, the anode and cathode initial substrate concentrations show linear relationship with product formation rate; however MES operation with a 2 day cycle time shows a more complex behaviour, with acetic acid production rates reaching a plateau and even a slight decrease at higher concentrations of the two substrates. It is also shown that there is a trade-off between product formation rate and substrate consumption and CE. MES performance for operation with cycle time being controlled by substrate consumption is also described. Results from the analysis demonstrate the interdependence of the system parameters and highlight the importance of multi-objective system optimization based on targeted end-use.


 Please wait while we load your content...
Please wait while we load your content...