Impact of charge state on 193 nm ultraviolet photodissociation of protein complexes†
Abstract
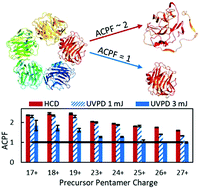
As applications in mass spectrometry continue to expand into the field of structural biology, there have been an increasing number of studies on noncovalent biological assemblies. Ensuring that protein complexes maintain native-like conformations and architectures during the transition from solution to the gas phase is a key aim. Probing composition and arrangement of subunits of multi-charged complexes via tandem mass spectrometry (MS/MS) may lead to protein unfolding and the redistribution of charges on the constituent subunits, leading to asymmetric charge partitioning and ejection of a high-charged monomer. Additionally, the overall dissociation efficiency of many ion activation methods is often suppressed for low charge states, hindering the effectiveness of MS/MS for complexes that have low charge density. Ultraviolet photodissociation (UVPD) of proteins using 193 nm photons is a high-energy alternative to collisional activation and demonstrates little to no charge state dependence. Here the symmetry of charge partitioning upon UVPD is evaluated for an array of multimeric protein complexes as a function of initial charge state. The results demonstrate that high laser energies (3 mJ) for UVPD induces more symmetric charge partitioning and ejection of low-charged, presumably compact monomers than higher-energy collisional dissociation (HCD).
- This article is part of the themed collection: 2019 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...