Conversion mechanism of enoyl thioesters into acyl thioesters catalyzed by 2-enoyl-thioester reductases from Candida Tropicalis†
Abstract
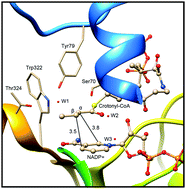
Enoyl thioester reductase from Candida tropicalis (Etr1p) catalyzes the NADPH-dependent conversion of enoyl thioesters into acyl thioesters, which are essential in fatty acid and second metabolite biosynthesis. In this paper, we explored the detailed catalytic mechanism of Etr1p by performing QM/MM calculations. Here, we focused on the formation of the covalent ene adduct intermediate and the proton transfer from Tyr79 to the substrate. Our calculation results reveal that the formation of the stable covalent ene adduct follows the Michael addition mechanism rather than the electrocyclic ene reaction. In addition, the ene adduct intermediate can reversibly decompose into the carbanion, and the proton of Tyr79 undertakes a direct electrophilic attack on the substrate to yield the product. In addition, three crystal water molecules do not participate in the catalytic reaction, but they play a crucial role in the hydride transfer and the proton transfer processes by forming a hydrogen bond network. These findings presented here would benefit our understanding of the catalytic mechanism of the NADPH-dependent enzyme.


 Please wait while we load your content...
Please wait while we load your content...