Pd local structure and size correlations to the activity of Pd/TiO2 for photocatalytic reforming of methanol†
Abstract
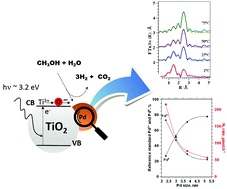
The interaction between Pd and TiO2 for promoting photocatalytic activity was investigated by tailoring the size of Pd nanoparticles and monitoring the photocatalytic activity of methanol photo-reforming reaction for hydrogen gas production. We show that at 0.6 wt% Pd loading, the catalyst with highly dispersed nanoparticles obtained at 1 °C temperature exhibits superior photocatalytic activity for hydrogen gas production. At different weights of Pd loading, tailoring two sets of catalysts with different structural properties provides correlation between the changes in the Pd local structures and the rate of hydrogen production. The impact of controlling the structural properties of metal nanoparticles on influencing H2 production outweighs the effect of metal loading variation. The differences of Pd/TiO2 activity at the different metal loadings were correlated with the changes in the Pd local structure consequently affecting the electronic transfer and photocatalytic efficiency.


 Please wait while we load your content...
Please wait while we load your content...