Rigidified and expanded N-annulated perylenes as efficient donors in organic sensitizers for application in solar cells†
Abstract
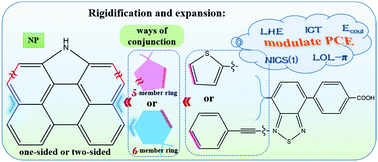
A class of N-annulated perylene (NP)-based organic dyes used in dye-sensitized solar cells has been investigated by means of quantum chemical calculations. The NPs are rigidified with thiophene or benzene rings in both a one-sided and two-sided manner on a 5- or 6-member ring and are considered as electron donors in dyes. To gain a better understanding of the effect of such modulation of NP moieties on the dye performance, the geometrical and electronic structures, the optical absorption and intramolecular charge transfer properties of the dyes and dye–TiO2 complexes are analyzed in detail to establish the structure–property relationship. The calculated results indicate that the rigidified NP moieties could improve light-harvesting capacities, modulate the energy levels of frontier orbitals, accelerate intramolecular charge transfer by decreasing the aromaticity of the π-system, decreasing the reorganization energy and avoiding electon trapping in the possible multiple electron transfer pathways, and facilitate charge separation with a lower coulombic attractive energy. In particular, the bilateral dyes via a 6-member immobilization would be promising candidates with excellent performance. We hope that our calculations could give a more in-depth physical insight on the structure–property relationship and provide guidance for the exploration of high-performance NP-based dyes.


 Please wait while we load your content...
Please wait while we load your content...