Effects of single and double nickel doping on boron clusters: stabilization of tubular structures in BnNim, n = 2–22, m = 1, 2
Abstract
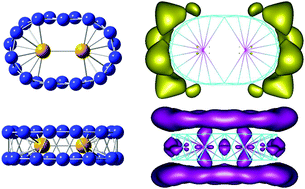
A systematic investigation on structure, relative stabilities, dissociation behavior and bonding of the singly and doubly Ni doped boron clusters BnNim with n = 2–22 and m = 1–2, was carried out using density functional theory (TPSSh functional) calculations. Calculated results indicate that for n < 14, BnNim structures are generally formed by capping Ni atom(s) on the edge or the surface of the pure boron Bn frameworks. From n = 14, the Ni dopants exert stronger effects in such a way that the most stable isomers BnNim adopt the shape of the related double ring tubular boron structures. With n ≥ 20, the Bn double ring appears to possess a large enough volume to entirely enclose the Ni2 dimer. The B14Ni and B22Ni2 turn out to be remarkable species with enhanced thermodynamic stability with larger average binding energies along with surprising geometric structures. Their higher thermodynamic stability can be understood in terms of the MO energy levels predicted by a hollow cylinder model, and other electronic properties. The (2 0 2)-orbital derived from the model of particle in a hollow cylinder appears to play a key role in the stabilization of the boron double ring.


 Please wait while we load your content...
Please wait while we load your content...