The multiple dissociation constants of glutathione disulfide: interpreting experimental pH-titration curves with ab initio MD simulations†
Abstract
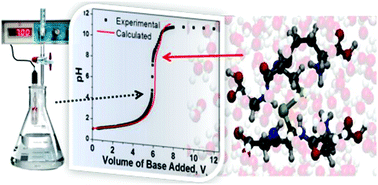
The hexapeptide glutathione disulfide (GSSG) has six ionizable groups with six associated dissociation constants. The experimentally measured pH-titration curve, however, does not exhibit the six corresponding equivalence points and bears little resemblance to standard textbook examples of acid–base pH-titration curves. The curve highlights the difficulties in determining multiple pKa values of polyprotic acids – typically proteins and peptides – from experiment. The six pKa values of GSSG can, however, be estimated using Car–Parrinello molecular dynamics (CPMD) simulations in conjunction with metadynamics sampling of the underlying free energy landscape of the dissociation reactions. Ab initio MD simulations were performed on a GSSG molecule solvated by 200 water molecules. Using the estimated pKa values the theoretical titration curve was calculated and found to be in good agreement with experiment. The results clearly highlight how dissociation constants estimated from ab initio MD simulations can facilitate the interpretation of the pH-titration curves of complex chemical and biological systems.
- This article is part of the themed collection: 2019 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...