The effect of co-adsorbed solvent molecules on H2 binding to metal alkoxides†
Abstract
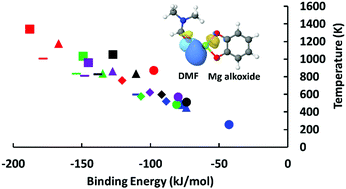
The introduction of metal alkoxides has been proposed as an attractive option to enhance hydrogen binding energies in porous materials such as metal–organic frameworks (MOFs) for room-temperature hydrogen storage applications. The presence of residual solvent molecules from MOF synthesis can, however, affect the performance of these functional groups. We perform quantum chemical calculations to predict solvent binding energies onto the metal-alkoxides and the temperatures required to drive off the solvent molecules and successfully activate porous materials with these moieties. Calculations are performed for Li, Mg, Zn, Cu, and Ni alkoxides and chloroform (CHCl3), dimethylformamide (DMF), ethanol, methanol, and water solvent molecules. We identify CHCl3 as a promising solvent that can be removed from these alkoxides at mild temperatures, whereas DMF binds strongly to the metal alkoxides and removal would require temperatures above the present upper bound of thermal stability in MOFs. As a second objective, we calculated the binding energies of hydrogen to metal alkoxide–solvent complexes to explore the effect of any solvent molecules that cannot be removed.
- This article is part of the themed collection: 2019 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...