Height-driven structure and thermodynamic properties of confined ionic liquids inside carbon nanochannels from molecular dynamics study†
Abstract
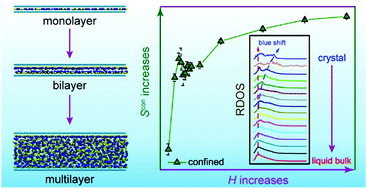
Understanding the structural transition of ionic liquids (ILs) confined in a nanospace is imperative for the application of ILs in energy storage, gas separation, and other chemical engineering techniques. In this work, the quantitative relations between the properties and height of the nanochannel (H) for the ([Emim]+[TF2N]−) IL are explored through molecular dynamics simulations. Interestingly, the entropy of the confined IL exhibits a nonmonotonic behavior as H increases: initially increasing for H < 1.0 nm and then decreasing for 1.0 < H < 1.1 nm, followed by increasing again for H > 1.1 nm; it finally approaches that of liquid bulk ILs. The vibrational spectrum of the confined IL is analyzed to investigate the nature of nonmonotonic entropy, showing that the liquidity and partial solidity will be respectively attenuated and enhanced as H decreases from 5.0 to 0.75 nm. Moreover, the hydrogen bond (HB) network and external force are also calculated, showing similar nonmonotonic behaviors when compared with the thermodynamic properties. The entropy gain of the confined IL originates from the reduced HB interactions, weaker external force, and partial solid nature, where more phase space sampling for ILs inside a bilayer graphene nanochannel (BLGC) can be achieved. All the above relations demonstrate that there exists a critical height of the nanochannel (HCR = 1.0 nm) at which the confined IL possesses weaker HB interaction, higher entropy, and better stability. The critical height of the nanochannel is also identified in the analysis of the local structures of cation head groups and anions, indicating that the confined IL could have a faster in-plane diffusive ability. These factors can serve as key indicators in quantitatively characterizing the mechanism for the structural transition of ILs inside a nanochannel and facilitate the rational design of nanopores and nanochannels to regulate the properties and structures of ILs in practical application scenarios.


 Please wait while we load your content...
Please wait while we load your content...