Conformational equilibria in o-anisic acid and its monohydrated complex: the prevalence of the trans-COOH form†
Abstract
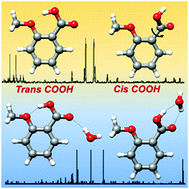
The conformation of ortho-anisic acid and its monohydrated clusters generated in a supersonic jet has been studied by rotational spectroscopy to analyze the structural implications of ortho substitution. The dominant monomer species shows a trans-COOH arrangement stabilized by an intramolecular O–H⋯O hydrogen bond from the acid to the methoxy group. The spectra of two non-planar skeleton monomer forms with a cis-COOH arrangement have also been detected. A tunneling doublet has been observed for the most stable cis-COOH conformer. The periodic potential energy function for the COOH internal rotation connecting these cis forms has been estimated from the experimental data. For the first time a water complex with an acid in a trans-COOH configuration has been observed. This is more abundant than the other weak form observed for the complex, characterized by a cis-COOH arrangement. The observation of the rotational spectra of salicylic acid, methyl salicylate and methyl 2-methoxybenzoate, formed upon heating of ortho-anisic acid, gives additional interest in the chemistry of ortho substituted benzoic acids.
- This article is part of the themed collection: 2019 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...