A rational design of manganese electrocatalysts for Lewis acid-assisted carbon dioxide reduction†
Abstract
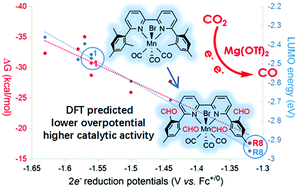
Herein, the mechanisms of Brønsted acid- and Lewis acid-assisted CO2 electroreduction by Mn(mesbpy)(CO)3Br (1) were investigated by density functional theory calculations. Our results indicate that for the Lewis acid-assisted cycle, an energy sink (13) is present owing to the interaction between Mg(OTf)2 and activated CO2, which is disadvantageous to the apparent activation energy (ΔG≠). Moreover, a series of substituted 13 counterparts were investigated to reduce the energy sink and decrease ΔG≠. Based on our study on the substituent effect, an excellent linear relationship was found between 2e reduction potentials and LUMO energies of substituted 1, and a moderate linear relationship was observed between ΔG of substituted 13 and the 2e reduction potential of substituted 1 counterparts. Moreover, for the CO2 reduction assisted by a Lewis acid, the formyl-substituted complex R8 has been predicted to be a more effective catalyst with lower overpotential and higher catalytic activity than its parent complex 1.


 Please wait while we load your content...
Please wait while we load your content...