Gas-phase reactivity of CH3OH toward OH at interstellar temperatures (11.7–177.5 K): experimental and theoretical study†
Abstract
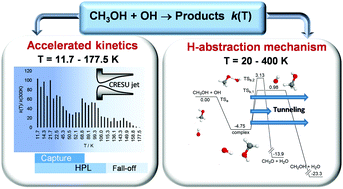
The reactivity of methanol (CH3OH) toward the hydroxyl (OH) radical was investigated in the temperature range 11.7–177.5 K using the CRESU (French acronym for Reaction Kinetics in a Uniform Supersonic Flow) technique. In the present study, the temperature dependence of the rate coefficient for the OH + CH3OH reaction, k(T), has been revisited and additional experimental and computational data are reported. New kinetic measurements were performed to fill the existing gaps (<22 K, 22–42 K and 88–123 K), reporting k(T < 20 K) for the first time. The lowest temperature ever achieved by a pulsed CRESU has been obtained in this work (11.7 K). k(T) abruptly increases by almost 2 orders of magnitude from 177.5 K to around 100 K. At T < 100 K, this increase is less pronounced, reaching the capture limit at temperatures below 22 K. The pressure dependence of k(T) has been investigated for selected temperatures and gas densities (1.5 × 1016 to 4.3 × 1017 cm−3), combining our results with those previously reported. No dependence was observed within the experimental uncertainties below 110 K. The high- and low-pressure rate coefficients, kHPL(T) and kLPL(T), were also studied in detail using high-level quantum chemical and theoretical kinetic methodologies, closely reproducing the experimental data between 20 and 400 K. The results suggest that the experimental data are near the high pressure limit at the lowest temperatures, but that the reaction remains a fast and effective source of CH2OH and CH3O at the low pressures and temperatures prevalent in the interstellar medium.


 Please wait while we load your content...
Please wait while we load your content...