Evaluation of the effect of nickel clusters on the formation of incipient soot particles from polycyclic aromatic hydrocarbons via ReaxFF molecular dynamics simulations†
Abstract
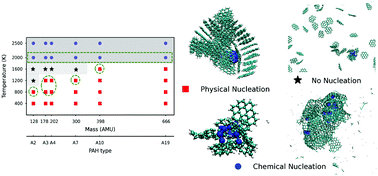
In the present study, the ReaxFF reactive molecular dynamics simulation method was applied to investigate the effect of a small nickel cluster (Ni13) on the formation of nascent soot from polycyclic aromatic hydrocarbon (PAH) precursors. A series of NVT simulations was performed for systems of a Ni13 cluster and various PAH monomers, namely, naphthalene, anthracene, pyrene, coronene, ovalene, and circumcoronene, at temperatures from 400 to 2500 K. At low temperatures, the PAHs form soot particles via binding and stacking around nickel clusters. Larger soot particles are formed due to the early initiation of clustering provided by nickel compared to those observed in homogenous PAH systems. At 1200–1600 K, the PAH monomers show a chemisorption tendency onto the nickel surface, which results in incipient soot particles. Chemical nucleation was observed at 2000 K where nickel-assisted dehydrogenation and chemisorption of PAH led to the growth of stable soot particles, which did not occur in the absence of Ni-clusters. At a high temperature (2500 K), nickel significantly accelerates the ring-opening and graphitization of PAH molecules and increases the size of the fullerene-type soot as compared to that of homogenous systems.


 Please wait while we load your content...
Please wait while we load your content...
