Investigation of ECD conformational transition mechanism of GLP-1R by molecular dynamics simulations and Markov state model†
Abstract
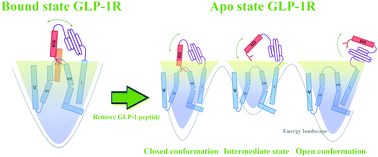
As a member of the class B G protein-coupled receptors (GPCRs), the glucagon-like peptide-1 (GLP-1) can regulate the blood glucose level by binding to the glucagon-like peptide-1 receptor (GLP-1R). Since the extracellular domain (ECD) of GLP-1R is considered as one of the binding sites of GLP-1, the open and closed states of ECD play an important role in the binding process of GLP-1. To investigate the transition path of GLP-1R ECD, the crystal structures of GLP-1R in its bound and unbound states (apo-state) are chosen to perform a total of 1.6 μs of molecular dynamics simulations. The simulated results show that the ECD of GLP-1R closes in the GLP-1 bound state and opens in the GLP-1 unbound state. To determine the critical role that GLP-1 played in regulating the open and closed states of the ECD, we applied the independent gradient model (IGM) to the simulation trajectories. We found that the “hand-like” N-terminal of the GLP-1R ECD plays an important role in the GLP-1 binding. In contrast, the apo-state GLP-1R ECD opens and exposes the two ligand binding domains of GLP-1 after 200 ns of simulations. To elucidate the open and closed mechanisms of GLP-1R ECD in the apo-state and GLP-1 bound state, the Markov state model (MSM) is performed on the MD simulation trajectories. Our results provide possible transition pathways from the closed state to open state of the apo-state GLP-1R ECD. Each pathway contains several intermediate states that correspond to different local minima in deep wells. The dynamical relationships and the most possible conversion pathway between two states are detailed through the MSM analysis. Our results profile the conformation transition mechanism of the GLP-1R ECD and will help in hypoglycemic peptide design of GLP-1R.


 Please wait while we load your content...
Please wait while we load your content...