Cost-effective approach to detect Cu(ii) and Hg(ii) by integrating a smartphone with the colorimetric response from a NBD-benzimidazole based dyad†
Abstract
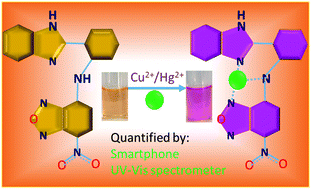
A new optical chemosensor N1 was designed and synthesized by condensing 4-chloro-7-nitrobenzofurazan with 2-aminophenylbenzimidazole. In CH3OH : H2O (1 : 1, v/v) medium, sensor N1 exhibited high selectivity and sensitivity towards Cu2+ and Hg2+ ions by showing a distinct colour change from pale yellow to pink due to the internal charge transfer occurring between the sensor N1 and the Cu2+/Hg2+ ions upon complexation in 1 : 1 stoichiometry. Also, the binding of Cu2+ and Hg2+ ions with N1 resulted in new absorption bands at 540 nm and 375 nm with the concurrent disappearance of the sensor absorption bands at 485 nm and 321 nm. Using the spectral changes of N1, the concentrations of Cu2+ and Hg2+ ions can be detected down to 1.23 × 10−7 M and 4.70 × 10−7 M, respectively. Further, the colour change of N1 in the presence of Cu2+/Hg2+ ions was integrated with a smartphone colour-scanning app to measure the red–green–blue (RGB) colour intensity, and a cost-effective method was developed for the on-site detection of Cu2+/Hg2+ ions. Finally, the practicability of sensor N1 to quantify Cu2+ and Hg2+ ions in real water samples was successfully validated by using both the UV-vis spectrophotometer and the smartphone.


 Please wait while we load your content...
Please wait while we load your content...