Molecular insight into chymotrypsin inhibitor 2 resisting proteolytic degradation†
Abstract
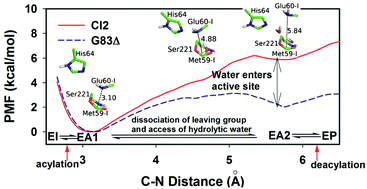
Chymotrypsin inhibitor 2 (CI2) is a special serine protease inhibitor which can resist hydrolysis for several days with a rapid equilibrium between the Michaelis complex and acyl-enzyme intermediate. The energies and conformational changes for subtilisin-catalyzed proteolysis of CI2 were examined in this paper for the first time by employing pseudo bond ab initio QM/MM MD simulations. In the acylation reaction, a low-barrier hydrogen bond between His64 and Asp32 in the transition state together with the lack of covalent backbone constraints makes the peptide bonds of CI2 break more easily than in other serine protease inhibitors. After acyl-enzyme formation, molecular dynamics simulations showed that the access of hydrolytic water to the active site requires partial dissociation of the leaving group. However, retention of the leaving group mainly by the intra- and inter-molecular H-bonding networks hinders the access of water and retards the deacylation reaction. Instead of the dissociation constant of inhibitors, we suggest employing the free energy at the acyl-enzyme state to predict the relative hydrolysis rates of CI2 mutants, which are testified by the experimental relative hydrolysis rates.


 Please wait while we load your content...
Please wait while we load your content...