Cation influence on heterocyclic ammonium ionic liquids: a molecular dynamics study†
Abstract
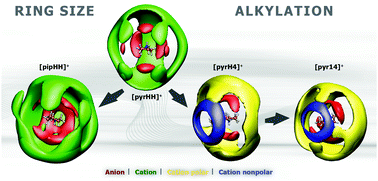
Four different ionic liquids (ILs) consisting of the bis(trifluoromethanesulfonyl)imide ([NTf2]−) anion, with structurally similar systematically varying cations, are investigated herein through classical molecular dynamics. The following cations were examined: pyrrolidinium ([pyrHH]+), piperidinium ([pipHH]+), N-butyl-pyrrolidinium ([pyrH4]+) and N-butyl-N-methyl-pyrrolidinium ([pyr14]+). The focus herein is on understanding the effect of increased ring size and alkyl chain addition, resulting in three protic ILs and one aprotic IL ([pyr14][NTf2]), on the physicochemical properties of the liquids studied herein. Addition of alkyl groups to the cation appears to cause a distinct weakening of inter-ionic interactions and ordering in comparison to increasing the ring size. The influence of these structural changes, however, is clearer on the ordering of like ions than oppositely charged ions. The protic ILs exhibit important similarities in the spatial arrangement of ions on account of their strong and directed H-bonding interactions. The cation is seen to influence particular conformations of the anion which further explains the more selective ordering in the protic ILs. However, the aggregation of the butyl side chain is also seen to be an important structural determinant in [pyrH4][NTf2] and [pyr14][NTf2]. We analyze the formation of domains in order to quantitatively evaluate the microheterogeneity arising in these systems from the separation of phases according to polarities. Velocity autocorrelation functions are studied in order to characterize the stronger caging effect in the protic ILs and the weakening of the caging effect upon addition of alkyl groups to the cation, these are consistent with the coordination environment within the respective liquids. In conclusion, significant correlations between the structure and properties of these ILs are observed and quantified within this contribution.


 Please wait while we load your content...
Please wait while we load your content...