Kinetics of the reactions of NO3 radical with alkanes†
Abstract
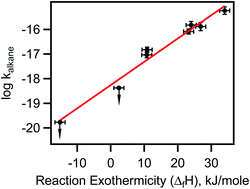
The rate coefficients for the reactions of NO3 radicals with methane (CH4), ethane (C2H6), propane (C3H8), n-butane (n-C4H10), iso-butane (iso-C4H10), 2,3-dimethylbutane (C6H14), cyclopentane (C5H10) and cyclohexane (C6H12) at atmosphere pressure (1000 ± 5 hPa) and room temperature (298 ± 1.5 K) were measured using an absolute method. Careful attention was paid to the role of secondary reactions and impurities. The upper limits of rate coefficients for methane and ethane at 298 K are <4 × 10−20 and <5 × 10−19 cm3 molecule−1 s−1, respectively. The rate coefficients at 298 K for propane, n-butane, iso-butane, 2,3-dimethybutane, cyclopentane and cyclohexane are, (9.2 ± 2.9) × 10−18, (1.5 ± 0.4) × 10−17, (8.2 ± 2.2) × 10−17, (5.8 ± 2.4) × 10−16, (1.5 ± 0.6) × 10−16 and (1.3 ± 0.4) × 10−16 cm3 molecule−1 s−1, respectively. Rate coefficients for the reactions of NO3 radical with two deuterated n-butanes (butane-D10 and butane-1,1,1,4,4,4-D6) are also reported. We show that the rate coefficients for NO3 reactions correlate with the enthalpy change for the reaction, thereby suggesting that the mechanism for NO3 reactions with alkanes is through H atom abstraction. The measured rate coefficients are compared with available literature values. This study increases the number of available rate coefficients for the reactions of NO3 with alkanes and sets significantly lower upper limits for reaction of NO3 with ethane and methane. The atmospheric significance of our reported rate coefficients is briefly discussed.
- This article is part of the themed collection: 2019 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...