Thermodynamics and kinetics of an oxygen adatom on pristine and functionalized graphene: insight gained into their anticorrosion properties
Abstract
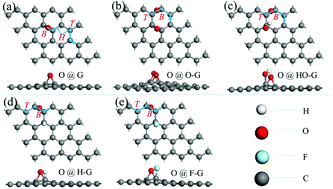
The thermodynamic and kinetic stabilities of an O adatom on graphene are critical factors for the formation of oxide defects in graphene, which leads to the breakdown of a graphene protective coating. To systematically understand various behaviors of an O adatom on graphene under the space conditions, the adsorption energies, diffusion paths and barriers, and penetration paths and barriers of the O adatom on pristine and functionalized graphene (e.g., –O, –OH, –H, and –F) are calculated using density functional theory, and the electronic structures are also analyzed in depth to reveal the microscopic mechanisms. We find that chemical functionalization increases both the adsorption stability and diffusion mobility of the O adatom on graphene, implying the possibly exacerbated destructive oxidation and even breakdown of the graphene-based coating. Furthermore, the penetration of the O adatom through pristine and functionalized graphene is also calculated, the occurrence of which is proved to be impossible in reality due to the associated extremely high energetic barriers. The calculated results, revealed mechanisms, and the gained insight into the corrosion resistance of graphene will be helpful for the design, synthesis, and application of related graphene-based protective coatings.


 Please wait while we load your content...
Please wait while we load your content...