Monitoring solvent dynamics and ion associations in the formation of cubic octamer polyanion in tetramethylammonium silicate solutions†
Abstract
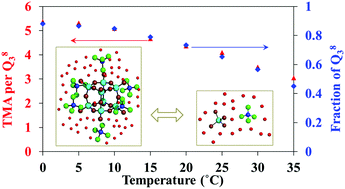
NMR methods were utilized to monitor the in situ structural and dynamic changes of various species in highly alkaline tetramethylammonium (TMA) silicate solutions. Quantitative 29Si NMR, 1H, 2H, and 17O relaxation NMR, and 1H and 29Si diffusion NMR of silicates, TMA, H2O and D2O demonstrate that the growth of the cubic octamer Q38 is accompanied by reduced water mobility and increasing TMA coordination number per Q38, which reaches an equilibrium value of 4.5 at 15 °C. Temperature-dependent measurements further reveal that the increased control over speciation by TMA at lower temperatures results from the more stable ion associations via slower solvent motions.


 Please wait while we load your content...
Please wait while we load your content...